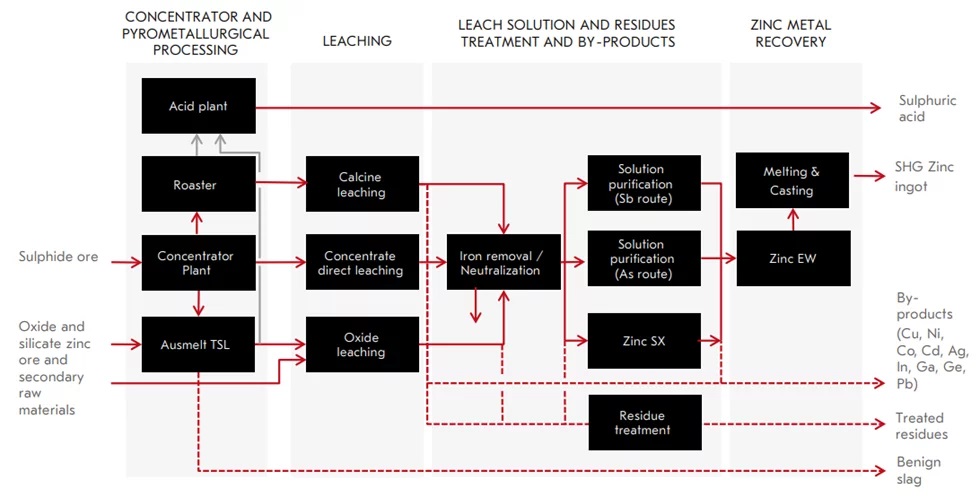
The Hydrometallurgical Process for Zinc Recovery
Zinc-Containing Industrial Wastes
Zinc-containing industrial wastes can originate from industries such as galvanizing, metallurgy, scrap recycling, and steel production. These wastes often contain zinc in the form of zinc oxide, zinc compounds with iron, or other metals.
Leaching Solutions (Lixiviants)
The hydrometallurgical process uses various leaching solutions, such as sulfuric acid, hydrochloric acid, ammoniacal solutions, or sodium hydroxide (NaOH), to dissolve zinc from the waste materials. The choice of lixiviant depends on the composition of the waste and the level of impurities.
- Sulfuric Acid (H₂SO₄): This is the most commonly used lixiviant, suitable for dissolving zinc oxides from EAF dust or other zinc-rich scraps. The product of this leaching process is typically zinc sulfate solution, which can be further refined and electrolyzed to recover metallic zinc.
- Hydrochloric Acid (HCl): Often used when it is necessary to dissolve heavy metals like lead or cadmium along with zinc. HCl can dissolve certain impurities that sulfuric acid cannot handle effectively.
- Ammoniacal Solutions: These are used to leach zinc from high-impurity sources such as sludge or slag. Ammonia-based solutions can selectively dissolve zinc while minimizing the dissolution of other metal impurities.
Purification and Zinc Recovery
Once zinc is leached into the solution, the solution must be purified to remove impurities like iron, lead, copper, and manganese. Common purification methods include:
- Precipitation: Chemical reagents are used to precipitate impurities out of the solution. For example, iron can be precipitated as hydroxide by increasing the solution’s pH.
- Ion Exchange or Solvent Extraction: More advanced chemical methods are employed to selectively remove specific metals from the solution, allowing for the separation of zinc from other impurities.
- Electrolysis: After purification, zinc can be recovered as a pure metal through the electrolysis of the zinc sulfate solution.
Applications and Advantages of the Hydrometallurgical Method
The hydrometallurgical process for zinc recovery from industrial wastes presents several applications and notable advantages across various industries. Here’s a detailed breakdown:
Applications of the Hydrometallurgical Process
-
Zinc Recovery from Industrial Wastes
- The hydrometallurgical method is particularly useful for recovering zinc from wastes such as Electric Arc Furnace (EAF) dust, zinc ash, and slags from galvanizing processes. These wastes contain valuable zinc compounds that can be efficiently processed and reused, rather than being discarded as hazardous materials.
-
Treatment of Low-Grade Zinc Ores
- This method is also applied to zinc recovery from low-grade ores, which are not economically viable for conventional pyrometallurgical methods. By using leaching, even ores with low zinc content can be exploited, contributing to more sustainable resource utilization.
-
Processing of Zinc-Containing Secondary Materials
- Hydrometallurgical techniques are effective for recovering zinc from various secondary materials such as batteries, alloys, and electronic waste. The selective leaching of zinc makes it an excellent choice for urban mining and recycling of electronic components.
-
Production of High-Purity Zinc Compounds
- The process is often used to manufacture high-purity zinc sulfate or zinc oxide, which are essential in industries like agriculture (as fertilizers) and pharmaceuticals (as additives). The high purity achieved via purification stages enhances the quality and usability of zinc in these sectors.
Advantages of the Hydrometallurgical Process
-
Lower Energy zinc recovery method
- Compared to pyrometallurgical methods, which require extremely high temperatures, hydrometallurgy operates at much lower temperatures. This leads to a significant reduction in energy consumption, making it more cost-effective and environmentally friendly.
-
Reduced Environmental Impact
- The hydrometallurgical method produces fewer air emissions and less slag, unlike pyrometallurgy, which generates large amounts of CO2 and toxic fumes. The controlled chemical reactions in the hydrometallurgical process minimize environmental hazards, particularly in terms of air and soil contamination.
-
Selective Metal Recovery
- One of the major advantages of hydrometallurgy is its ability to selectively recover zinc while leaving other metals behind. Through precise pH adjustments and the use of selective reagents, impurities like lead, cadmium, and iron are removed during the purification stages, allowing for the production of high-purity zinc.
- Recovers by-products such as Pb, Cu, Cd, Co, Ni, In, Ga, Ag, Au, and Ge.
-
Ability to Treat Complex Materials
- The hydrometallurgical process can handle complex waste materials with multiple metal compositions. By tailoring the leaching conditions, it is possible to target zinc recovery while minimizing the dissolution of unwanted metals. This is especially useful when dealing with multi-metallic industrial wastes.
-
Economic Viability for Low-Grade Ores and Wastes
- The cost-effectiveness of hydrometallurgy lies in its ability to recover zinc from low-grade ores and industrial wastes that would otherwise be uneconomical to process using traditional methods. This opens up opportunities for industries to monetize materials that are often considered waste, promoting a circular economy.
-
Scalability and Flexibility
- Hydrometallurgical processes are highly scalable, suitable for both large industrial operations and smaller, more specialized recovery systems. This flexibility allows companies to tailor the process to their specific volume and material requirements, making it a versatile solution across various industries.
In Summary of Hydrometallurgical Process for Zinc Recovery
The hydrometallurgical method offers a sustainable and efficient solution for recovering zinc from industrial wastes and low-grade ores. Its lower energy consumption, minimal environmental impact, and ability to selectively recover high-purity zinc make it a preferred method in modern zinc recycling processes. The adaptability of the technique to treat complex and secondary materials further enhances its appeal, driving its widespread application in industries focused on sustainability and resource conservation.
Visit Metso for Hydrometallurgy solutions.


